Company
Vidipharm was incorporated in January 2013 by three co-owners and has developed and registered Diamucin™, a ready to ship quantitative breast cancer test kit.
At the moment more than 20 million women are diagnosed with breast cancer in the European Union alone. To control and adjust a given therapy, testing twice or three times per year is recommended and ethically justifiable. The primary goal of this follow-up monitoring is early detection prior to the emergence of disease recurrence and the appearance of daughter tumors (metastases) and to prompt the initiation of a changed treatment.
At a time of distinct awareness for breast cancer screening and follow-up, Diamucin™ provides information about the effectiveness of the current breast cancer treatment. The treating physician can control or alter the deployed therapy, which will lead to a significant increases in life expectancy by timely adjustment of a given therapy.
The target group consists of women who are already diagnosed with breast cancer and their physicians. As there is no other quantitative breast cancer specific test kit on the market, first surveys indicate a very positive resonance and acceptance of the patients as well as physicians.
Taking into account the heterogeneous insurance structures in the EU countries our medium-term objective is to gain more than 60 % market share until 2020. As the intellectual property rights of the test kit are secured internationally, sales might be expanded beyond Europe as well.
Further R&D activities will concentrate on gaining the same level of information for other types of epithelium originated types of cancer, e.g. lung or colorectal cancer as well as from peripheral blood. For that a cooperation treaty was signed with an Austrian university.
As the test kit is classified as a medical device, no time consuming and expensive clinical tests are necessary. However, as a marketing instrument, we plan to give away 16.000 test kits for free in Austria in the first year. This covers about all new cases of breast cancer in Austria and will motivate the state health insurance to provide the same level of control for the already existing base of 340.000 breast cancer patients.

Product
Diamucin TM (MTL-HEP for Breast Cancer Patients) is a quantitative tumor cells receptors expression measurement system developed for initial diagnostic as well as monitoring patient’s cancer remission/progression in dynamics.
It is aimed to replace existing routine hormone-sensitive cancer’s diagnostic such as hormone receptors immune precipitation/ histochemistry and tumors cytology-microscopy analysis but has the difference with the delivery of dynamic alterations results for personalized medical treatment suggestion to both doctor and patient.
Background: Antibodies-based immunohistochemistry diagnostics are routinely made tests for the expression absence-presence of hormone receptors and other cancer-associated antigens. However, these methods are not quantitative and cannot be applied in advanced patients monitoring and the adjustment of individual aromatase inhibitors/chemo- and radiotherapy regimes.
Precise quantification of main cancer cell receptors expression instead of “a lot”, “less than a lot” or “absence” makes targeting these receptors therapies quick adjustment possible.
Diamucin MTL-HEP test is quick, quantitative, less expensive and able to fulfill main tasks for biopsy/surgery breast cancer diagnostics. Quantitative measurement of ER1, PR, and HER2-neu expression levels monitors the chances of cancer progression in the individual dynamic and the fastest adjustment in adjuvant treatment for patients’ benefit.
High levels of Muc1 hyperexpression in triple-negative breast tumor patients as well as those ER-PR-negative patients undergoing aromatase inhibitor treatment are suitable for Muc1-specific therapeutic antibodies and anti-tumor Muc1 immunizing peptides (imMucin). The high ratio of malignant Muc1 isoforms in tumor samples indicates that these breast cancer patients provide the target for efficient PI3K inhibitor, (TOR) neo-adjuvant treatment.
recognize complex correlations
implementing in targeted manner
generate benefits
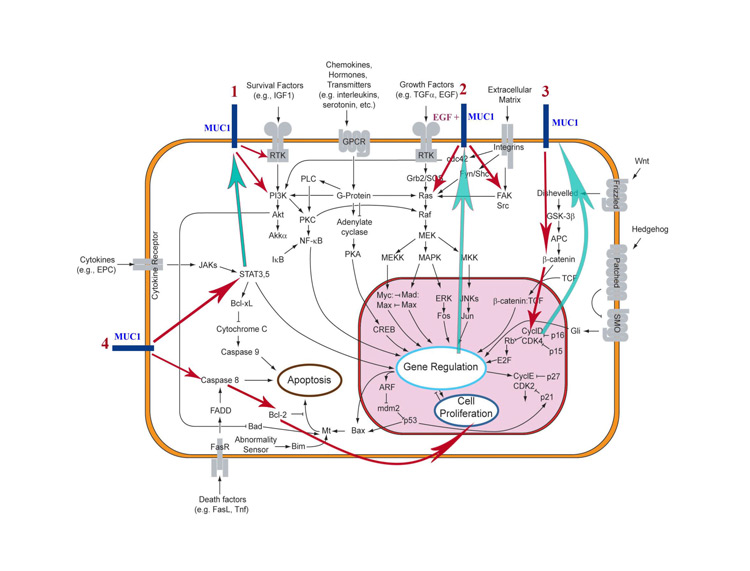
MUC1 Action in Normal and Cancer Cell Signal Transduction Pathways – Diagnostic Keys for Personalized Clinical Therapy Application.
In case Muc1 hyperexpression is a choice for treatment its activity should be blocked from the ”two ends” – by the inhibitor working inside signal pathway in combination with blockader of Muc1 receptor and co-receptor activity, either RTK (TK inhibitor) or therapeutic antibodies. Modern clinical trials analysis shows that normally antitumor generics do not have high enough therapeutic activity and, like most of chemotherapeutic medicines, are used in combinations in order to receive a synergetic effect and maximum DPF response. Unfortunately, tyrosine kinases inhibitors, which block Muc1 receptor phosphorylation activity and PI3K, TORC, MEK, STAT-Jak, CDK4/6, are also kinases inhibitors. So, their acute and chronic toxicities can often start in synergetic reaction, as well as their therapeutic effects. That is why we think it is for patient’s benefit to select these “two ends” inhibitors from different classes of medicines, for example, any kinase inhibitor and therapeutic mAbs, kinase inhibitor and aromatase inhibitor. The other crucial knowledge about anti-Muc1 therapy is the great variability of its malignant isoforms, so treatment with anti-Muc1 humanised monoclonal antibodies probably cannot bring as much as VEGF, EGFR1, HER2 therapeutic mAbs.
There are several attempts to make agents for the other types of MUC1-targeting medicines, binding its cellular domain, ligands to extracellular EGF-1-2 complexes. However, these developments are still far from demonstration any stable therapeutic effect.
EGFR1-MUC1 unit RTK tyrosine kinases inhibitors is a broad family of agents with a broad anti-RTK activity. In general, generics from this family are much less specialized for a certain ferment